95.011: Propylene Glycol vs. Ethylene Glycol Antifreeze
Introduction:
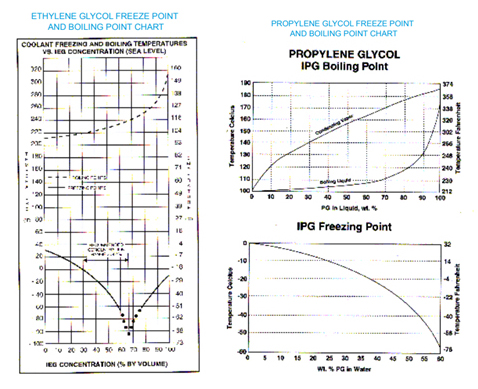
From time to time the Tech Service Department receives requests for information on the freeze points and boiling points of various dilutions or Ethylene and Propylene Glycol coolants.
Background:
The generally recommended and most common practice in the use of glycol based antifreezes is to use 50 vol% water and 50 vol% antifreeze The water should be pure water, such as deionized or distilled water.
In some instances, a fleet may desire to run a weaker (more water) coolant to improve heat exchange and/or reduce antifreeze expense. This practice results in reduced corrosion inhibitor concentration, poorer freeze protection and slightly lower boiling points. The inhibitors can be fortified using additives such as Pencool® 3000. The boiling point reduction may result in undetected coolant boiling, since many engine temperature warning devices are set at 260°F, and weak coolants will boil before they reach this temperature.
Freeze protection, as the graphs illustrate, show that maximum protection is achieved with Ethylene Glycol Coolants at 67% antifreeze and Propylene Glycol coolants at 100% antifreeze. Customers are strongly advised to consult with their vehicle and/or engine manufacturer before exceeding 67% glycol concentrations. Heat exchange properties are significantly different at higher concentrations. Ethylene Glycol concentrations above 67% are counterproductive and are not recommended. The following graphs offer useful information.